Product Info Summary
| SKU: | AR0106 |
|---|---|
| Size: | 1 kit |
Product info
Kit Components
| Description | Quantity | Volume | Catalog Number |
| Cytoplasmic Extraction Reagent A (CER A) | 1 | 30mL | AR0106-A |
| Cytoplasmic Extraction Reagent B (CER B) | 1 | 1.5mL | AR0106-B |
| Nuclear Extraction Reagent (NER) | 1 | 15mL | AR0106-C |
Overview
| Product Name | Cytoplasmic and Nuclear Protein Extraction Kit |
|---|---|
| KU/Catalog Number | AR0106 |
| Form | Liquid |
| Pack Size | 1 kit |
| Assays per kit | 60 assays for cell pellet fractions having packed cell volumes of 50µL each 30 assays for 0.1g tissue |
| Storage | Upon receipt store Cytoplasmic and Nuclear Protein Extraction Kit at 4°C. It is stable at 4°C for one year. Product is shipped at ambient temperature. |
| Compatibility with reagents | Fully compatible with Broad Spectrum Protease Inhibitor Cocktail and Broad Spectrum Phosphatase Inhibitor Cocktail |
| Equivalent | Thermofisher (Product No. 78833, 78835), Millipore Sigma (Product No. NXTRACT) |
| Description | Boster’s Cytoplasmic and Nuclear Protein Extraction Kit provides a complete set of extraction reagents that enable the separation of nuclear protein and cytoplasmic fractions from cultured cells and fresh tissues. |
| Cite This Product | Cytoplasmic and Nuclear Protein Extraction Kit (Boster Biological Technology, Pleasanton CA, USA, Catalog # AR0106) |
Assay Principle
This kit provides a complete set of extraction reagents that enable the separation of nuclear protein and cytoplasmic fractions from cultured cells and fresh tissues. The kit breaks cell membrane and release cytoplasmic proteins for cell burst under hypotonic condition. And then centrifuge for collection of the nucleoli. At last, extract nuclear proteins using Nuclear Extraction Reagent. Once desalted or diluted, the isolated soluble cytoplasmic proteins can be used to perform immunoassays and protein interaction experiments, such as EMSA, Co-IP and pull-down assays. Nuclear extracts are generally preferred to whole cell lysates for gene regulation studies. Cellular components present in whole cell lysates can adversely affect nuclear protein interactions and stability, and nuclear proteins are more concentrated in nuclear extracts than whole cell lysates.
Features of the Cytoplasmic and Nuclear Protein Extraction Kit:
Compatible—Extracted proteins can be directly apply for downstream assays, including Western blotting, gel-shift assays, protein assays, reporter gene assays and enzyme activity assays.
Fast—The optimized reagents and protocol allow non-denatured, active proteins to be purified in 90 minutes.
Convenient—simple instructions do not require ultracentrifugation over gradients.
Minimum cross-contamination—Cross-contamination of cytosolic proteins into the membrane fractions is usually about 10%.
Important Product Information
1. All steps of protein extraction should be operated on ice or at 4°C.
2. The kit is designed for fresh tissue samples only. It will not work efficiently for frozen tissue samples.
3. Use BCA protein Assay kit (Product No. AR0146) to quantify isolated proteins.
4. If more concentrated nuclear extracts are desired, the volume of NER used in the extractions can be decreased 2- to 4-fold without adverse effects on protein recovery or compartmentalization.
5. If large volumes of nuclear extract are required in subsequent applications or if problems occur with downstream assays, dialyze the nuclear extract to remove excess salts before use.
6. Include protease inhibitors to maintain extract integrity and function.
Additional Materials Required
• Protease inhibitor (Product No. AR1182) and phosphatase inhibitor (Product No. AR1183)
• 2mL microcentrifuge tubes
• Vortex mixer
• Microcentrifuge capable of spinning at 16,000 x g
• Tissue homogenizer
• Phosphate-buffered saline (PBS): 0.1M phosphate, 0.15M sodium chloride; pH 7.2
Procedure for Cytoplasmic and Nuclear Protein Extraction from Different Sample Types
Reagent Preparation:
Place Cytoplasmic Extraction Reagent A (CER A), Cytoplasmic Extraction Reagent B (CER B) and Nuclear Extraction Reagent (NER) on ice. For optimal results, include protease inhibitor and phosphatase inhibitor before use.
Protocol 1: Adherent Cells & Suspension Cells
Cell Culture Preparation
1. For adherent cells: scrape the cells off the surface of the plate with a cell scraper. Centrifuge harvested cells at 600 x g for 5 minutes. Carefully remove and discard the supernatant, and keep cell pellets for use.
2. For suspension cells: centrifuge harvested cells at 600 x g for 5 minutes. Carefully remove and discard the supernatant, and keep cell pellets for use.
3. Resuspend the cells in pre-cooling PBS.
4. Transfer the cells to a 2mL microcentrifuge tube. Centrifuge at 600 x g for 5 minutes. Carefully remove and discard the supernatant, and keep cell pellets for use.
5. Add CER A to the cell pellet according to the volumes indicated in Table 1.
Table 1. Reagent volumes for different packed cell volumes
| Packed cell Volume (μL) | CER A (μL) | CER B (μL) | NER (μL) |
| 10 | 100 | 5 | 50 |
| 20 | 200 | 10 | 100 |
| 50 | 500 | 25.5 | 250 |
| 100 | 1000 | 50 | 500 |
The volume of 2×106 Hela cells is about 20μL.
6. Vortex the tube at maximum speed for 15 seconds to obtain a homogeneous cell suspension. Incubate on ice for 10-15 minutes.
7. Add CER B to the tube. Vortex the tube at maximum speed for 5 seconds. Incubate on ice for 1 minute. (If there is cytoplasmic protein in extracted nuclear protein, prolong the vortex time by 1-5 minutes.)
8. Vortex the tube at maximum speed for 5 seconds. Centrifuge the tube at 16000 × g for 5 minutes.
9. Immediately transfer the supernatant containing cytoplasmic proteins to a clean pre-chilled tube. Place this tube on ice until use or store aliquots at -80°C for future use.
10. Add NER to the insoluble cell debris containing nuclei produced in step 8.
11. Vortex at maximum speed for 5 seconds to obtain a homogeneous cell suspension (If not complete, prolong the vortex time). Place the cell debris on ice and continue vortexing for 15 seconds every 10 minutes, for a total of 40 minutes.
12. Centrifuge the tube at 16000 × g for 5 minutes.
13. Immediately transfer the supernatant containing nuclear proteins to a clean pre-chilled tube. Place this tube on ice until use or store aliquots at -80°C for future use.
Protocol 2: Tissue
Tissue Preparation:
1. Place the fresh tissue into pre-chilled PBS and rinse several times. Dry the tissue with filter paper. Mince the tissue into small pieces and weigh the tissue sample.
2. Place tissue in a tissue homogenizer. Add CER A to the tissue according to the volumes indicated in Table 2.
Table 2. Reagent volumes for different tissue amounts
| Tissue Weight (mg) | CER A (μL) | CER B (μL) | NER (μL) |
| 20 | 200 | 10 | 100 |
| 40 | 400 | 20 | 200 |
| 80 | 800 | 40 | 400 |
| 100 | 1000 | 50 | 500 |
3. Homogenize tissue on ice to obtain a homogeneous suspension. Transfer the homogeneous suspension to a 2mL microcentrifuge tube. Incubate on ice for 10 minutes.
4. Add CER B to the tube. Vortex the tube at maximum speed for 5 seconds. Incubate on ice for 1 minute.
5. Vortex the tube at maximum speed for 5 seconds. Centrifuge the tube at 16000 × g for 5 minutes.
6. Immediately transfer the supernatant containing cytoplasmic proteins to a clean pre-chilled tube. Place this tube on ice until use or store aliquots at -80°C for future use.
7. Add NER to the insoluble cell debris containing nuclei produced in step 5.
8. Vortex at maximum speed for 5 seconds to obtain a homogeneous cell suspension (If not complete, prolong the vortex time). Place the cell debris on ice and continue vortexing for 15 seconds every 10 minutes, for a total of 40 minutes.
9. Centrifuge the tube at 16000 × g for 5 minutes.
10. Immediately transfer the supernatant containing nuclear proteins to a clean pre-chilled tube. Place this tube on ice until use or store aliquots at -80°C for future use.
Note:
For some tissues, if the cytoplasmic or nuclear proteins fail to be extracted as expected, follow Protocol 3.
Protocol 3: Tissue
1. Place the fresh tissue into pre-chilled PBS and rinse several times. Dry the tissue with filter paper. Mince the tissue into small pieces and weigh the tissue sample.
2. Place tissue in a tissue homogenizer.
3. Mix CER A with CER B at a volume ratio of 20:1 to generate CER A and CER B Mixture. e.g. Add 10μL of CER B into 200μL of CER A.
4. Add CER A and CER B Mixture to the tissue at a ratio of 10:1 (v/w). e.g. Add 200μL of CER A and CER B Mixture to 20mg tissue.
5. Homogenize tissue on ice to obtain a homogeneous suspension. Transfer the tissue homogenate to a 2mL microcentrifuge tube. Incubate on ice for 15 minutes.
6. Centrifuge the tube at 1500 × g for 5 minutes at 4 ˚C.
7. Immediately transfer the supernatant containing cytoplasmic proteins to a clean pre-chilled tube. Place this tube on ice until use or store aliquots at -80°C for future use. (These are partial cytoplasmic proteins extracted from the tissue sample. Do not touch the pellet when aspirating the supernatant. There are still many cells in the pellet that have not been broken.)
8. Add CER A to the cell pellet according to the volumes indicated in Table 3.
Table 3. Reagent volumes for different packed cell volumes
| Packed cell Volume (μL) | CER A (μL) | CER B (μL) | NER (μL) |
| 10 | 100 | 5 | 50 |
| 20 | 200 | 10 | 100 |
| 50 | 500 | 25.5 | 250 |
| 100 | 1000 | 50 | 500 |
The volume of 2×106 Hela cells is about 20μL.
9. Vortex the tube at maximum speed for 15 seconds to obtain a homogeneous cell suspension. Incubate on ice for 10-15 minutes.
10. Add CER B to the tube. Vortex the tube at maximum speed for 5 seconds. Incubate on ice for 1 minute.
11. Vortex the tube at maximum speed for 5 seconds. Centrifuge the tube at 16000 × g for 5 minutes.
12. Immediately transfer the supernatant containing cytoplasmic proteins to a clean pre-chilled tube. Place this tube on ice until use or store aliquots at -80°C for future use.
13. Add NER to the insoluble cell debris containing nuclei produced in step 8.
14. Vortex at maximum speed for 5 seconds to obtain a homogeneous cell suspension (If not complete, prolong the vortex time). Place the cell debris on ice and continue vortexing for 15 seconds every 10 minutes, for a total of 40 minutes.
15. Centrifuge the tube at 16000 × g for 5 minutes.
16. Immediately transfer the supernatant containing nuclear proteins to a clean pre-chilled tube. Place this tube on ice until use or store aliquots at -80°C for future use.
Note: Cytoplasmic proteins produced in Step 6 and Step 11 can be stored and used together.
Troubleshooting
| Problem | Possible Cause | Solution |
|---|---|---|
| Low cytoplasmic protein yield | Cells were not lysed completely Cell pellet was not dispersed Tissues was not homogenized sufficiently | Increase amount of CER A and CER B Reagent Vortex thoroughly Homogenize sufficiently |
| Low nuclear protein yield | Cell pellet was not dispersed ncomplete nuclei isolation | Vortex thoroughly Increase time of centrifugation |
| No or low protein activity detected | Samples were not kept cold Presence of protease | Keep samples on ice between vortexing steps Use a protease inhibitor cocktail |
| Proteins not compartmentalized | Extraction time for cytoplasmic protein is too long. ncomplete removal of cytoplasmic extract Cytoplasmic extract contains some nuclear precipitation while transferring the supernatant containing cytoplasmic proteins | Decrease extraction time for cytoplasmic protein. Carefully remove all cytoplasmic extract before nuclear lysis Carefully remove all cytoplasmic extract before nuclear lysis |
Related Boster Products
AR1182 Broad Spectrum Protease Inhibitor Cocktail
AR1183 Broad Spectrum Phosphatase Inhibitor Cocktail
AR0146 BCA Protein Assay Kit
AR0131 SDS-PAGE Loading Buffer (2×)
Product Images
Validation Images & Assay Conditions

Click image to see more details
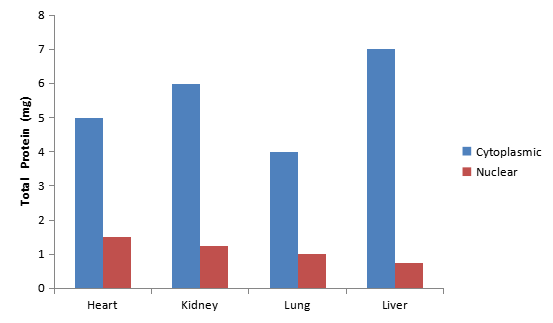
Click image to see more details
Nuclear proteins and cytoplasmic proteins were extracted from mouse heart, kidney, lung and liver tissues (100mg) with Boster Cytoplasmic and Nuclear Protein Extraction Kit. BCA protein Assay kit (Product No. AR0146) was used to quantify isolated nuclear and cytoplasmic proteins.

Click image to see more details
Nuclear proteins (NE) and cytoplasmic proteins (CE) were extracted from mouse heart, kidney, lung and liver tissues with Boster Cytoplasmic and Nuclear Protein Extraction Kit. Proteins were used to perform WB for GAPDH and PCNA expressed in cytoplasm and in nuclei.
Specific Publications For AR0106
Hello CJ!
AR0106 has been cited in 148 publications:
*The publications in this section are manually curated by our staff scientists. They may differ from Bioz's machine gathered results. Both are accurate. If you find a publication citing this product but is missing from this list, please let us know we will issue you a thank-you coupon.
Ozonated autohemotherapy elevates PPAR-γ expression in CD4+ T cells and serum HDL-C levels, a potential immunomodulatory mechanism for treatment of psoriasis
The role of RIP1 and RIP3 in the development of aplastic anemia induced by cyclophosphamide and busulphan in mice
Theranostic Nanodots with Aggregation-Induced Emission Characteristic for Targeted and Image-Guided Photodynamic Therapy of Hepatocellular Carcinoma
Estrogen receptor α/prolactin receptor bilateral crosstalk promotes bromocriptine resistance in prolactinomas
Ozone Therapy Attenuates NF-κB-Mediated Local Inflammatory Response and Activation of Th17 Cells in Treatment for Psoriasis
Expression and Distribution of the Guanine Nucleotide-binding Protein Subunit Alpha-s in Mice Skin Tissues and Its Association with White and Black Coat Colors
Porcine parvovirus nonstructural protein NS1 activates NF-κB and it involves TLR2 signaling pathway
Anti-inflammatory properties of lipoxin A4 protect against diabetes mellitus complicated by focal cerebral ischemia/reperfusion injury
Transcriptome changes induced by RUNX3 in cervical cancer cells in vitro
Resveratrol attenuates IL‑33‑induced mast cell inflammation associated with inhibition of NF‑κB activation and the P38 signaling pathway
Customer Reviews
Have you used Cytoplasmic and Nuclear Protein Extraction Kit?
Submit a review and receive an Amazon gift card.
- $30 for a review with an image
0 Reviews For Cytoplasmic and Nuclear Protein Extraction Kit
Customer Q&As
Have a question?
Find answers in Q&As, reviews.
Can't find your answer?
Submit your question
6 Customer Q&As for Cytoplasmic and Nuclear Protein Extraction Kit
Question
Are the nuclear membrane and nuclear envelope removed while using AR0106? Additionally, do these buffers contain SDS? What kind of detergents are used in the buffers supplied by this kit?
Verified customer
Asked: 2023-01-21
Answer
The nuclear membrane and nuclear envelope are not being removed when using the Cytoplasmic And Nuclear Protein Extraction Kit ( AR0106). Buffers in this kit don't contain SDS. The detergent used is NP-40.
Boster Scientific Support
Answered: 2023-01-30
Question
I ordered cytoplasmic and nuclear protein extraction kit with cartalog number AR0106. in the protocol it says that we also need phosphatase inhibitor. can I ask why do we need it?
Verified customer
Asked: 2022-07-18
Answer
Thank you for your question. Phosphatase inhibitor will be used if phosphorylated antibody is applied.
Boster Scientific Support
Answered: 2022-07-18
Question
Is AR0106 compatible with biorad assey?
Verified customer
Asked: 2021-02-26
Answer
Our lab hasn't used DC™ Protein Assay Kit II 5000112 from Biorad and we haven't validated if the Cytoplasmic And Nuclear Protein Extraction Kit (AR0106) is compatible with the kit. We suggest to run a pilot test and see if they are compatible.
Boster Scientific Support
Answered: 2021-02-28
Question
What is the composition of AR0106?
Verified customer
Asked: 2021-02-26
Answer
Unfiortunately, we can't disclose the composition of the Cytoplasmic And Nuclear Protein Extraction Kit (AR0106).
Boster Scientific Support
Answered: 2021-02-28
Question
What is the solution for problem with dissolving the pellet in the Nunclear Extraction Reagent even when increasing the vortex time and sufficient amount of the NER?
Verified customer
Asked: 2020-10-27
Answer
For the Cytoplasmic And Nuclear Protein Extraction Kit (AR0106), please continue to increase vortex time and strength.
Boster Scientific Support
Answered: 2020-10-28
Question
Can AR0106 be used with frozen cells or must they be freshly harvested?
Verified customer
Asked: 2019-07-15
Answer
The Cytoplasmic And Nuclear Protein Extraction Kit (AR0106) is designed for fresh tissue samples only. It will not work efficiently for frozen tissue samples.
Boster Scientific Support
Answered: 2019-07-15



