This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
Pro tips on resolving common Western Blot issues such as weak signal, wrong band size, smiley gel, and high background.
The following guide serves as a checklist for the possible causes and solutions to some of the most commonly encountered problems with Western blot assays.
If you do not see the issues you are having featured in this page, please contact us at [email protected] and we will help you resolve your specific trouble.
Download troubleshooting handbooks for IHC, Western blot, and ELISA for FREE.
Troubleshooting GuidesLet Bosterbio handle your Western blot CRO services and be your long term partner in broadening the bandwidth of your lab/team in a flexible manner.
High background on a western blot occurs when the background signal of the membrane reduces the signal-to-noise ratio to unreadable levels. Typically, polyacrylamide gel electrophoresis separates proteins by molecular weight, with large proteins migrating slower than small proteins. This process can be affected by several factors: protein degradation can cause proteins to appear shorter than the expected length, glycosylation can cause proteins to appear larger than predicted, and nonspecific antibody binding can cause multiple bands to appear on a blot where only one is expected.
Use these tips to identify and resolve the source of your unexpected band sizes.

| S.No. | Possible Cause | Solution |
|---|---|---|
| 1 | Antibody concentration is too high |
|
| 2 | Aggregate secondary antibody formation |
|
| 3 | Too high antibody incubation temperature |
|
| 4 | Non-specific secondary antibody binding or cross-reactivity with blocking agent |
|
| 5 | Cross-reactivity of primary or secondary antibody with blocking agent |
|
| 6 | Incompatible blocking agent |
|
| 7 | Incomplete blocking |
|
| 8 | Insufficient blocking |
|
| 9 | Cross-reactivity of antibody with other proteins |
|
| 10 | Insufficient washing |
|
| 11 | Exposure time is too long |
|
| 12 | Membrane problem |
|
| 13 | Insufficient membrane wash |
|
| 14 | Incompatible membrane |
|
| 15 | Dry membrane |
|
| 16 | Contaminated buffer |
|
| 17 | Contaminated equipment |
|
| 18 | Insufficient antibody binding activity |
|
| 19 | Excessive substrate incubation |
|
| 20 | Blocking proteins reacting with detection system |
|
A weak western blot signal is characterized by faint or indistinct bands. While the bands may be barely visible at their predicted sizes, weak signal can often require repeating the experiment. Common sources of this error occur during blocking, washing, protein transfer, or detection.
Use the tips below to identify the source of the error and get better results.
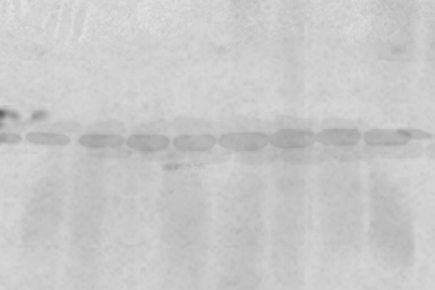
| S.No. | Possible Cause | Solution |
|---|---|---|
| 1 | Improper protein transfer to membrane |
|
| 2 | Insufficient protein and membrane binding |
|
| 3 | Insufficient antibody |
|
| 4 | Insufficient antigen |
|
| 5 | Antigen masking by blocking buffer |
|
| 6 | Presence of sodium azide in buffers |
|
| 7 | Too short exposure time |
|
| 8 | Too short substrate incubation time |
|
| 9 | Digestion of protein on membrane |
|
| 10 | Degradation of protein during storage |
|
| 11 | Incompatible primary and secondary antibodies |
|
| 12 | Low concentration of primary antibody and/or secondary antibody |
|
| 13 | Cross-reactivity between blocking agent and antibodies (primary or secondary) |
|
| 14 | Inability of primary antibody to recognize the protein in tested sample |
|
| 15 | Low or none content of target protein (ineffective antigen) |
|
| 16 | Insufficient transfer and excessive wash |
|
| 17 | Over-blocking |
|
| 18 | Loss of primary antibody effectiveness |
|
| 19 | Inhibition of secondary antibody by sodium azide |
|
| 20 | Loss of effectiveness in enzyme conjugate and substrate |
|
| 21 | Improper wet transfer for membrane |
|
| 22 | Insufficient molecular weight of target protein (< 10 kDa) |
|
| 23 | Equality or nearness in values between target protein’s isoelectric point and transfer buffer’s pH value |
|
| 24 | Too high methanol concentration |
|
| 25 | Insufficient sample concentration |
|
| 26 | Transfer too vigorous |
|
| 26 | Inadequate transfer |
|
| 27 | Sandwich assembly oriented incorrectly |
|
| 28 | Incorrect transfer buffer pH |
|
| 29 | Insufficient antibody binding affinity |
|
| 30 | Insufficient sample loading |
|
The background of a western blot does not always appear clean and flawless. Blotches, streaks, and spots are all common artifacts that can make it hard to interpret and publish your results. These artifacts are most commonly the result of uneven coating of buffer or antibody, the membrane drying out, or aggregates forming in the antibody or blocking buffer.
Follow the tips below to identify and solve the cause of your imperfect western blot background.
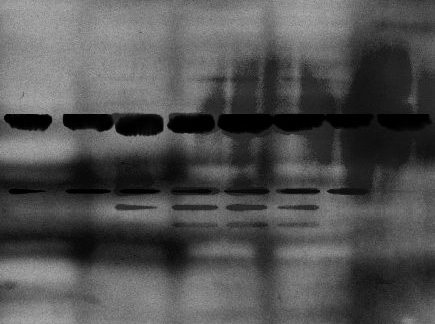
| S.No. | Possible Cause | Solution |
|---|---|---|
| 1 | Blotched background: Uneven antibody distribution |
|
| 2 | Blotched background: Membrane dried out unevenly |
|
| 3 | Blotched background: Uneven wash/incubation buffer coverage |
|
| 4 | Flecked background: Secondary antibody aggregation |
|
| 5 | Flecked background: Clumps of blocking buffer binding secondary antibody |
|
| 6 | Flecked background: Buffer contamination |
|
| 7 | White spots with no protein transfer: Air bubbles trapped between gel and membrane during transfer |
|
Typically, polyacrylamide gel electrophoresis separates proteins by molecular weight, with large proteins migrating slower than small proteins. This process is can be affected by several factors: protein degradation can cause proteins to appear shorter than the expected length, glycosylation can cause proteins to appear larger than predicted, and nonspecific antibody binding can cause multiple bands to appear on a blot where only one is expected.
Use these tips to identify and resolve the source of your unexpected band sizes.
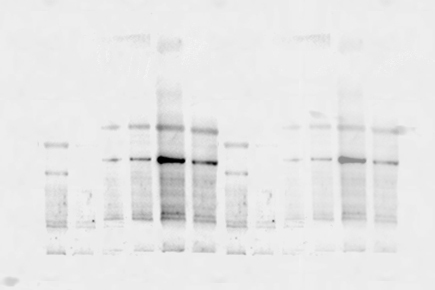
| S.No. | Possible Cause | Solution |
|---|---|---|
| 1 | Bands have higher MW than expected: Proteins are glycosylated or bear other post-translational modifications |
|
| 2 | Bands have much higher MW than expected: Protein aggregation |
|
| 3 | Bands have much higher MW than expected: Incomplete denaturation or residual disulfide bonding |
|
| 4 | Bands have lower MW than expected: Protein sample has been digested or degraded |
|
| 5 | Bands have lower MW than expected: Primary antibody is detecting splice variants |
|
| 6 | Bands have lower MW than expected: Primary antibody binding a similar epitope on a different protein |
|
| 7 | Multiple bands: Primary or secondary antibody contaminated with nonspecific IgG |
|
| 8 | Multiple bands: Nonspecific binding of primary antibody |
|
| 9 | Multiple bands: Nonspecific binding of secondary antibody |
|
| 10 | Multiple bands: Insufficient blocking |
|
| 11 | Multiple bands: Ionic interactions |
|
Distorted bands can make it very hard to interpret your results. Common distortions include smile-shaped bands with the edges trailing upward, diffuse bands that are broad or blurry, and streaked bands that trail off in several directions.
Make sure your next blot has even, crisp bands by following the tips below.
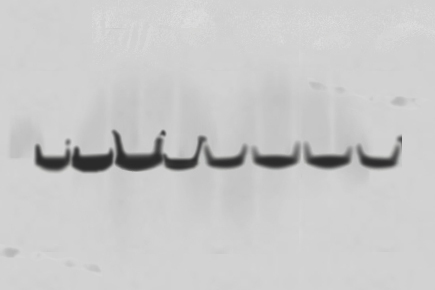
| S.No. | Possible Cause | Solution |
|---|---|---|
| 1 | Curved, "smiling" bands: Electrophoresis voltage too high |
|
| 2 | Curved, "smiling" bands: Overheated gel |
|
| 3 | Streaked or diffuse bands: Incomplete contact between gel and membrane during transfer |
|
| 4 | Streaked or diffuse bands: Slippage of membrane during transfer |
|
| 5 | Blurry bands: Electrophoresis voltage too high |
|
| 6 | Blurry bands: Improper loading buffer composition |
|
| 7 | Blurry bands: Air bubbles trapped between gel and membrane during transfer |
|
| 8 | Ghost bands: Overexposure during visualization |
|
| 9 | Ghost bands: Loading sample too concentrated |
|
| 10 | Ghost bands: Antibody concentration too high |
|
| 11 | Ghost bands: Blot was moved during transfer |
|
| 12 | Uneven, crooked bands: Poor gel polymerization |
|
| 13 | Uneven, crooked bands: Varying salt concentration between wells |
|
Unlock the full potential of your research and extract invaluable insights from your samples with our exceptional service. Don't hesitate—contact us now to discuss your project requirements and see how our expert Western blotting service can elevate your research.
![]()
Learn the concept behind Western blotting. It is a technique that is used to detect specific proteins in the given sample. It usually involves two major processes, namely, SDS-polyacrylamide gel electrophoresis and protein blotting and testing.
Learn about Western Blot Principle![]()
Check out this Western Blot sample preparation guides to learn how to get the best results from your sample type. Learn more about sample preparation in this guide.
Learn Western Blot Sample Preparation![]()
Learn a stepwise Western blotting protocol from reagent preparation to detection with application of BosterBio reagents. Check out our ELISA protocols to learn how to get the best results.
Check our Western Blot Protocol![]()
Get to learn the concept behind our best practises on Western Blot optimization. Learn how to optimize every aspect of your experiment to yield the best results.
Browse Western Blot Optimization Tips