This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
Every flow cytometry (or FACS) experiment begins with sample preparation. Check out our flow cytometry sample preparation guide to learn how to prepare your samples for flow cytometric analysis.
This information is to serve as a guide as individual investigators may need to optimize protocols for their particular cell type.
Cells for flow cytometry analysis are usually derived from four main sources:
Regardless of the source, the final cell preparation should be:
a homogenous single-cell suspension free of clumps and dead cell debris at a density of 106-107 cell per ml suspended in a suitable staining buffer.
PBMCs isolated from whole blood through Ficoll gradient centrifugation or RBC lysed whole blood or non-adherent cultured cells are readily available for flow cytometric analysis. Adherent cultured cells or cells present in the solid organs should be first made into a single cell suspension before flow analysis by using enzymatic digestion or mechanical dissociation of the tissue, respectively. Mechanical filtration should be followed to avoid unwanted instrument clogs or lower quality flow data. Use the following sample preparation protocols based on your appropriate starting materials.
Key Reagents – PBS, staining buffer
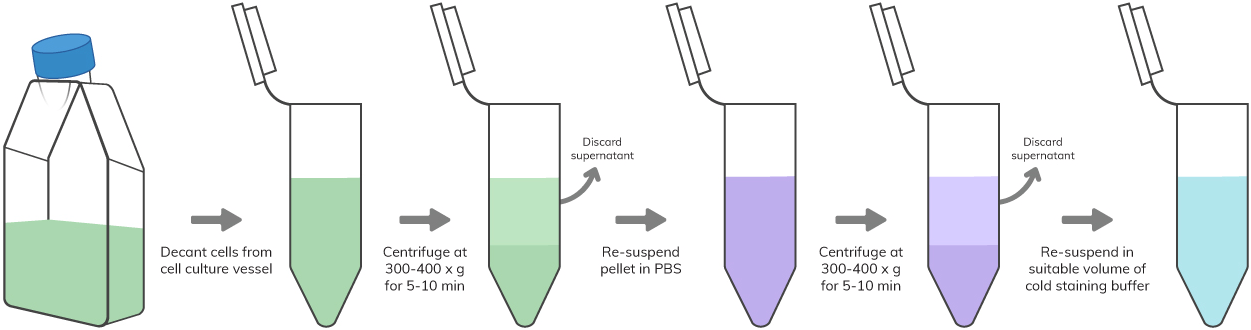
Key Reagents – PBS, staining buffer, 0.25% trypsin
Key reagents – PBS, staining buffer, culture medium with 10% FBS
Key reagents – PBS, staining buffers, suitable gradients medium like Ficoll or Histopaque
Key reagents – PBS, staining buffer (see appendix), RBC lysis buffer [check Appendix for recipe or use a commercially available buffer]
This guide will show you all the nuts and bolts for Flow Cytometry and FACS, including expert review of principle, optimized protocol that really works, and more.
Flow Cytometry Flow Cytometry Sample Preparation Optimization Sample preparation and cell quality tips Whenever possible, use freshly isolated cells rather than frozen and thawed cells. To increase viability of thawed cells, perform the initial dilut...
See MoreBoster Bio is an antibody company and supplier. Read more about our troubleshooting tips for flow cytometry (FACS) experiments. Learn tips on how to resolve issues such as high background, and weak or no signal
See MoreFlow cytometry is a popular cell biology technique that utilizes laser-based technology to count, sort, and profile cells in a heterogeneous fluid mixture. FACS is an abbreviation for fluorescence-activated cell sorting, which is a flow cytometry technique that further adds a degree of functionality.
See MoreBoster Bio protocols for flow cytometry offer a step-by-step overview of the procedure. Use this guide as a primer or a quick reference guide, and see our product datasheets or sample preparation guides for more details.
See MoreGet some of the best Bosterbio's flow cytometry optimization tips. This guide includes protocols, optimization tips, troubleshooting tips, and more on flow cytometry.
See MoreClick Here for More Flow Cytometry Sample Preparation Every flow cytometry (or FACS) experiment begins with sample preparation. Click Here for More Flow Cytometry Technical Blogs Here is our ever growing archive of technical blog articles related to ...
See MoreThis guide will show you all the nuts and blots for Flow Cytometry and FACS, including expert review of principle, optimized protocol that really works and more. Comprehensive Troubleshooting Tips Having our troubleshooting tips that address all kind...
See MoreBinding of antibody to surface antigen can stimulate the cells and alter the expression of intracellular signalling proteins. Saponin does not alter the surface antigen epitopes so surface staining can be done afterwards. Methanol is compatible with ...
See More