This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
We provide the step-by-step protocols for IHC-P, IHC-F, and ICC/IF to help you improve your assay performance and obtain clear result images.
Standardization is one of the most challenging aspects for the implementation of successful biospecimen staining. In an effort to accelerate your immunostaining of tissue sections and cell climbing slices, we have developed and validated our step-by-step IHC/ICC/IF protocols to cover biospecimen preparation and assay procedures. We believe these protocols will be a useful resource for your staining workflow or at least a good starting point for further protocol optimization if necessary. For each of the protocols, we also provide a summary flow chart with the applicable Boster’s reagents to enhance your product search on our website.
Get a unique combination of service quality, subject expertise and cost savings, when it comes to IHC service.
Boster manufactures IHC reagents that are used in our own validation processes. See the IHC protocol below with available Boster products highlighted.
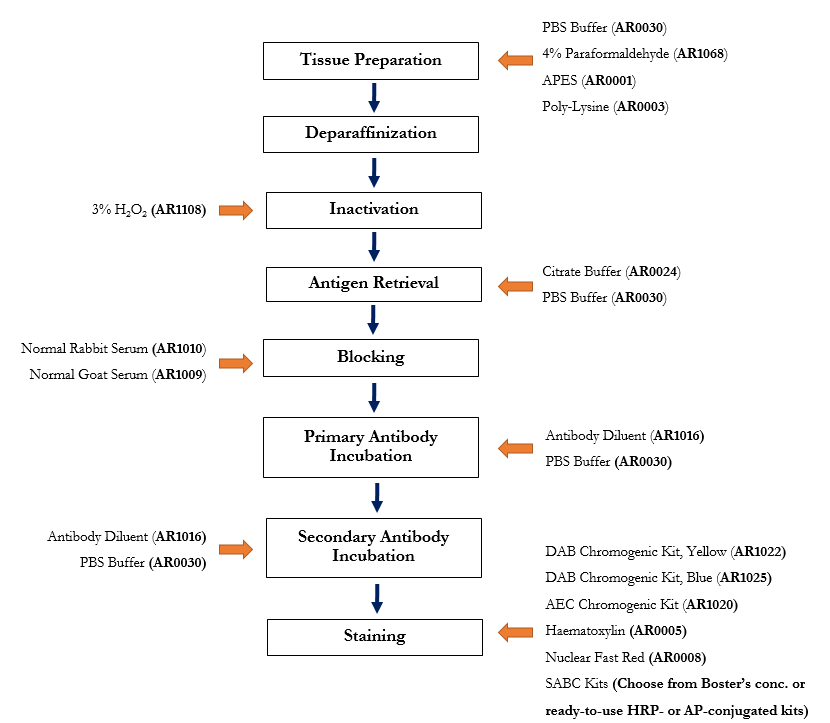
IHC Workflow (Paraffin-Embedded Sections) with Applicable Boster Reagents
You can save up to 90% on the above reagents if you buy them from Boster Bio
Step-by-step guide for IHC-P protocol with reagent recommendations
Used Products: PBS Buffer , 4% Paraformaldehyde
Note: This fixation procedure using paraformaldehyde and formalin fixatives may cause autofluorescence in the green spectrum. In this case, you may try fluorophores in the (i) red range or (ii) infrared range if you have an infrared detection system.
Prepare the following reagents:
Sequentially immerse paraffin sections into:
Note: The process of dewaxing should be done in a fume hood at room temperature in summer. When the temperature is lower than 18°C, it is recommended to dewax at 50°C
Used Products: 3% Hydrogen Peroxide
Used Products: Citrate Buffer Powder , PBS Buffer
Used Products: Normal Rabbit Serum
Used Products: Antibody Diluent , PBS Buffer
Used Products: Antibody Diluent , PBS Buffer
Used Products: DAB Chromogenic Substrate Kit (Brown) , DAB Chromogenic Substrate Kit (Blue) , AEC Substrate Kit , Hematoxylin , Nuclear Fast Red
*If the staining background is too high, wash the section 4X with 0.01-0.02% TWEEN 20 PBS and 2X with pure PBS after the SABC reaction and before DAB staining. Then use DAB to stain the samples.
Learn the probable causes and solutions of weak or no stainingBoster manufactures IHC reagents that are used in our own validation processes. See the IHC protocol below with available Boster products highlighted.
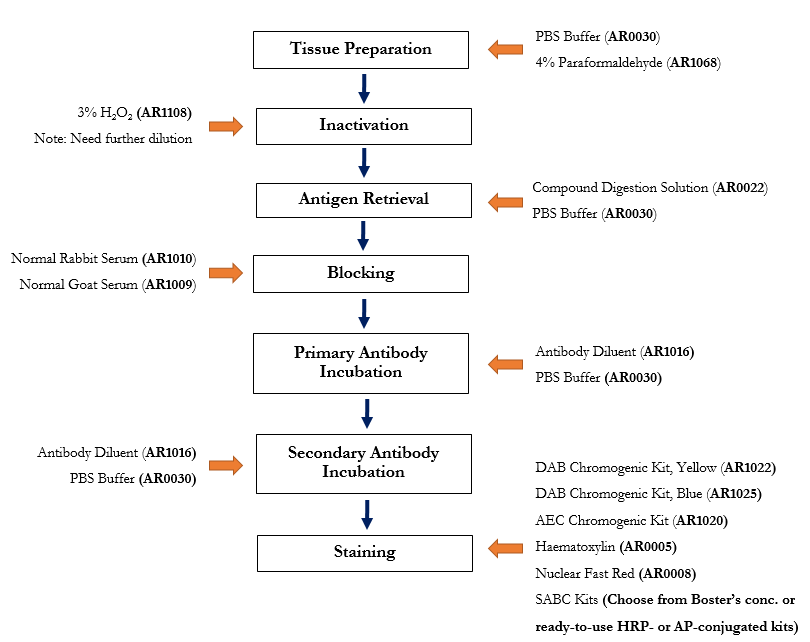
IHC Workflow (Frozen Sections) with Applicable Boster Reagents
Step-by-step guide for IHC-F protocol with reagent recommendations
Products Listed: PBS Buffer - 4% Paraformaldehyde
* The mold can simply be made by using tin foil
Products Listed: 3% H2O2
Products Listed: Enzyme Antigen Retrieval Reagent - PBS Buffer
Products Listed: 5% BSA blocking solution
Products Listed: Antibody Diluent - PBS Buffer
Products Listed: Antibody Diluent - PBS Buffer
DAB Chromogenic Kit, Yellow - AEC Chromogenic Kit - Haematoxylin - Nuclear Fast Red - PBS
Boster offers the full range of reagents one needs for performing smooth immunohistochemistry (IHC), immunofluorescence (IF), and immunocytochemistry (ICC) assays. See the protocol below with available Boster products highlighted.
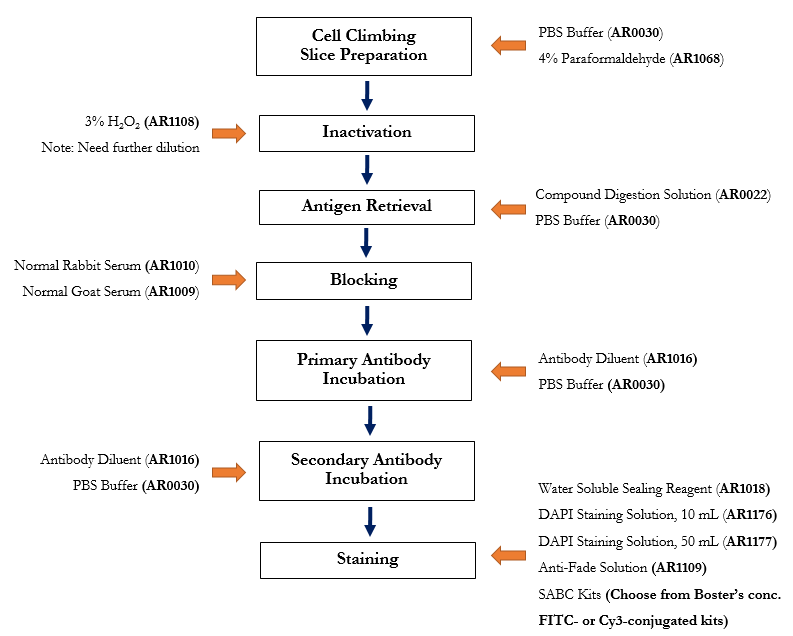
Immunocytochemistry (ICC) Workflow with Applicable Boster’s Reagents
Step-by-step guide for ICC/IF protocol with reagent recommendations
Products Listed: PBS Buffer - 4% Paraformaldehyde
Products Listed: 3% H2O2
Products Listed: Enzyme Antigen Retrieval Reagent - PBS Buffer
Read on how you can effectively select the right antigen retrieval method
Products Listed: 5% BSA blocking solution
Products Listed: Antibody Diluent - PBS Buffer
Products Listed: Antibody Diluent - PBS Buffer
Aqueous Mounting Medium - DAPI - DAPI - Antifade Mounting Medium -
Learn the probable causes and solutions of weak or no staining
The principle behind Immunohistochemistry (IHC) entails detection of antigen or happens in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. Learn more about this in this guide.
Learn the concept of IHC PrincipleIHC optimization is a critical step in any test. This guide gives you insight on antigen retrieval, fixation and embedding. Learn how to optimize your immunohistochemistry test to get valuable results.
Learn our IHC Optimization tipsThis guide provides a thorough list of Immunohistochemistry troubleshooting tips, including weak staining, high background, non specific staining among others. Learn how to take control of your IHC process..
Check our IHC troubleshooting tipsLearn the best IHC and ICC sample preparation techniques. Get a detailed procedure of preparing different types of preserved tissues which is key to getting high quality staining during Immunohistochemistry (IHC)..
Check out our IHC Sample Preparation