This website uses cookies to ensure you get the best experience on our website.
- Table of Contents
Boster Bio protocols for flow cytometry offer a step-by-step overview of the procedure. Use this guide as a primer or a quick reference guide, and see our product datasheets or sample preparation guides for more details.
Each human cell expresses hundreds of thousands of cell surface antigens that specify their cell type, biological function, development stage, and much more. Cells residing in different organs have characteristic cell surface antigens, and the determination of these cells using the specific fluorochrome-conjugated antibodies can be analyzed by flow cytometry. Staining with an unconjugated purified antibody needs an additional step of staining with a fluorescent conjugated secondary antibody (indirect immunostaining).
This information is to serve as a guide as individual investigators may need to optimize protocols for their particular cell type.
If the cells are stained in a 96 well U- or V-bottom plate, washing procedure should be set up first for maximum removal of unbound primary antibodies.
This guide will show you all the nuts and bolts for Flow Cytometry and FACS, including expert review of principle, optimized protocol that really works, and more.
Key Reagents – PBS, staining buffer, FACS buffer, PFA fixing buffer
Key Reagents – PBS, staining buffer, FACS buffer, PFAfixing buffer
Key Reagents – PBS, staining buffer, FACS buffer, 0.5-4% PFA in PBS [exact concentration of PFA has to be standardized for every antibody panel], 100% methanol
Key Reagents – PBS, staining buffer, FACS buffer, 0.5-4% PFA in PBS [exact concentration of PFA has to be standardized for every antibody panel], 0.1% saponin
Key Reagents – PBS, staining buffer, FACS buffer, 0.5-4% PFA in PBS [exact concentration of PFA has to be standardized for every antibody panel], 100% methanol
The intracellular staining procedure allows direct measurement of antigens (cytokines or transcription factors) present inside the cytoplasm or in the nucleus of a cell in addition to the surface antigen determination simultaneously. In this procedure, the fixation and permeabilization of cells are required after staining with fluorescently conjugated surface antigens. This modified staining procedure allows direct measurement of functional activity of any cell of interest present in the blood or other tissues without further separation. To achieve better results, additional in vitro cell stimulation with some common mitogen (e.g., PMA, Ca++ or peptide epitopes and protein transport inhibitor, Brefeldin A, etc.) may be required, which allows increased production of cytokines inside cells. Refer to the table below as a guideline for common cell stimulation procedures.
Note: If the cells are stained in a 96 well U- or V-bottom plate, washing procedure should be set up first for maximum removal of unbound primary or secondary antibodies.
| Target cytokine/phosphoprotein | Target cells | Stimulant | Duration | Surface marker |
|---|---|---|---|---|
| IL-2 | PBMCs | PMA (50ng/ml) | 4-6 hours | CD3 |
| IL-3 | T-cells | PMA(50ng/ml) + ionomycin (1µg(ml) | 4-6 hours | CD4 |
| IL-4 | PBMCs | PMA(50ng/ml) + ionomycin (1µg(ml) | 4-6 hours | CD4 |
| IL-6 | PBMCs | LPS (100ng/ml) | 4-6 hours | CD14 |
| IL-10 | PBMCs | LPS (100ng/ml) | 18-24 hours | CD14 |
| GM-CSF /IFNγ/TNFα/TNFβ | PBMCs | PMA(50ng/ml) + ionomycin (1µg(ml) | 4-6 hours | CD3 |
| pStat5 | PBMCs | GM-CSF (20ng/ml) + IL3 (20ng/ml) | 15 min. | CD123, CD116 |
| pStat3 | PBMCs | G-CSF (20ng/ml) + IL6 (20ng/ml) | 15 min. | CD126, CD114 |
| pERK | PBMCs | IL3 (20ng/ml) + IL6 (20ng/ml) + FLT3L (20ng/ml) | 15 min. | CD123, CD126, CD135 |
Dye exclusion staining is performed to separate live and dead cells, as well as to isolate the rare stem cell ‘side populations’. If viability staining is included in your regular immunostaining, it should be performed before any other staining.
Key reagents – PBS, staining buffer, PI solution (10µg/ml in PBS)
Key reagents – PBS, staining buffer, 7-AAD solution (100µg/ml in PBS)
Key reagents – PBS, 5% FBS in PBS, staining buffer, Hoechst 33342 solution (1mM in PBS) or Rho123 solution (10µg/ml in PBS)
Key reagents – PBS, staining buffer, PI solution (50µg/ml in PBS), RNAse A (10µg/ml), 70% ethanol
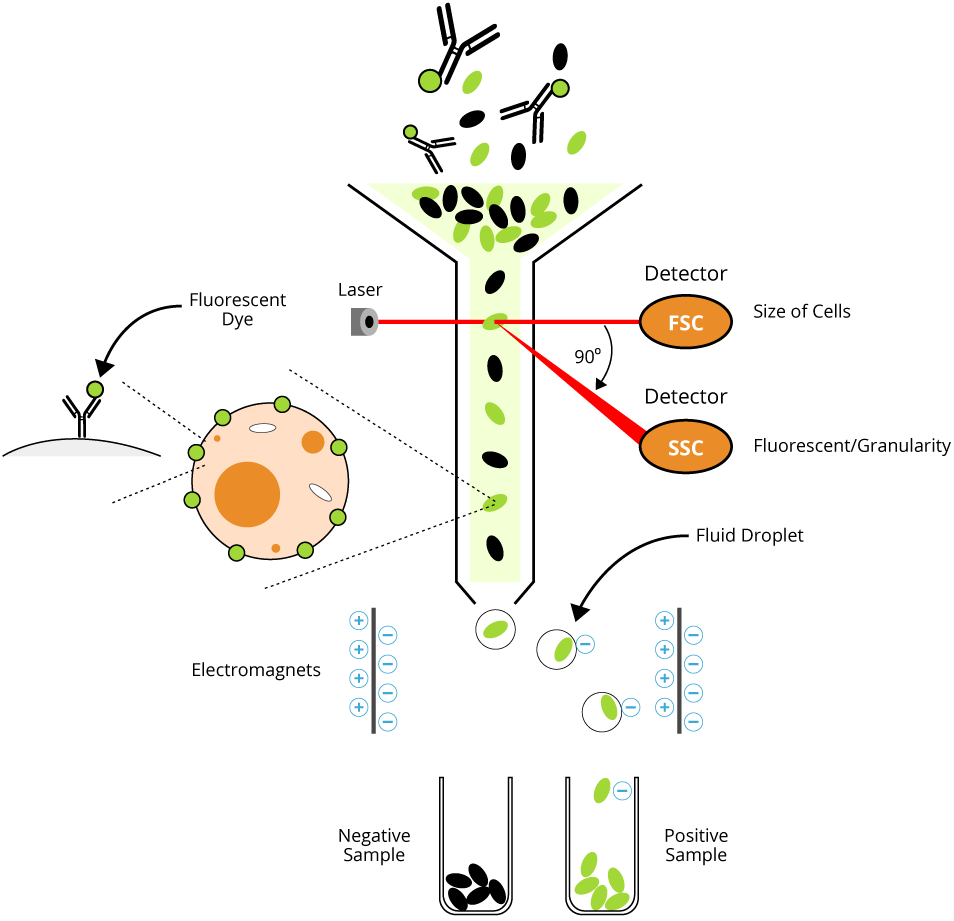
Flow cytometry is a popular cell biology technique that utilizes laser-based technology to count, sort, and profile cells in a heterogeneous fluid mixture. FACS is an abbreviation for fluorescence-activated cell sorting, which is a flow cytometry technique that further adds a degree of functionality.
See MoreBoster Bio is an antibody company and supplier. Read more about our troubleshooting tips for flow cytometry (FACS) experiments. Learn tips on how to resolve issues such as high background, and weak or no signal
See MoreEvery flow cytometry (or FACS) experiment begins with sample preparation. Check out our flow cytometry sample preparation guide to learn how to prepare your samples for flow cytometric analysis.
See MoreGet some of the best Bosterbio's flow cytometry optimization tips. This guide includes protocols, optimization tips, troubleshooting tips, and more on flow cytometry.
See MoreFlow Cytometry Flow Cytometry Sample Preparation Optimization Sample preparation and cell quality tips Whenever possible, use freshly isolated cells rather than frozen and thawed cells. To increase viability of thawed cells, perform the initial dilut...
See MoreBinding of antibody to surface antigen can stimulate the cells and alter the expression of intracellular signalling proteins. Saponin does not alter the surface antigen epitopes so surface staining can be done afterwards. Methanol is compatible with ...
See MoreAntibody titration is recommended to determine the correct concentration of an antibody for the optimum signal. Test different dilutions of the antibody to zero in on the lowest concentration that gives the strongest signal in positive control and th...
See MoreIn the case of staining for secreted cytokines, add brefeldin A or monensin during the incubation period at the concentration recommended by the manufacturer.
See More